Roche's Paroxysmal Nocturnal Hemoglobinuria Treatment Has EU Approval
Prior approvals for PiaSky (crovalimab) include those for monthly subcutaneous use in the US and Japan, where users with sufficient training may be able to self-administer the medication
Roche announced in a press release on Aug. 27, 2024 that PiaSky (crovalimab) has received approval from the European Commission (EC) for specific patients with paroxysmal nocturnal hemoglobinuria (PNH). PiaSky is a monoclonal antibody that helps inhibit the complement protein C5. It has been approved by the EC for use in adults and adolescents aged 12 and older, who weigh at least 40 kg (approximately 88 pounds). This treatment is suitable for individuals who are new to or have previously received C5 inhibitors.
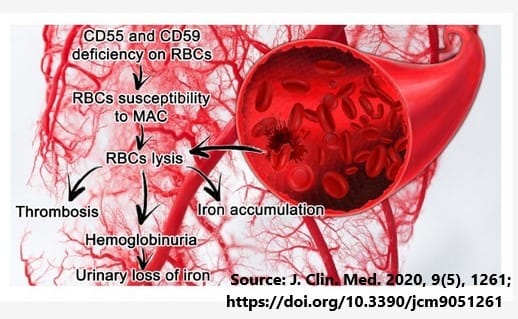
PNH is a rare and life-threatening blood condition characterized by the complement system, which is part of the innate immune system, attacking and destroying red blood cells. These symptoms can include anemia, fatigue, and blood clots, and may even progress to kidney disease.
According to Roche, the recycling technology utilized in the creation of PiaSky enables the medicine to be administered subcutaneously on a monthly basis. This method allows for a smaller volume of medicine to effectively bind and inhibit the C5 protein multiple times, resulting in a longer-lasting effect in the body. This is the first monthly subcutaneous treatment for PNH that has been approved in the European Union, United States, and Japan. Patients now have the option to self-administer after receiving proper training.
In a Phase III study called COMMODORE 2, researchers examined the use of crovalimab in people with PNH who had not received C5 inhibitors before. The study found that the rate of adverse events in patients treated with crovalimab (78%) was comparable to that of eculizumab, which is a standard C5 inhibitor used in current treatment.
According to Alexander Röth, MD, the lives of people with PNH can be greatly impacted by the need for frequent intravenous infusions and time-consuming clinic visits. This not only affects their own lives but also the lives of their caregivers and families. The demands of treatment can become a central focus for them. It is crucial to provide individuals with PNH the ability to have more control over their treatment and greater independence. This can be achieved through the use of PiaSky, a treatment option that is equally effective but requires less frequent administration and can be conveniently given at home.
"The approval of PiaSky introduces a fresh option to the treatment options for PNH. It combines the ability to control the disease through C5 inhibition with an innovative recycling technology that allows for monthly subcutaneous administration," stated Levi Garraway, MD, PhD, Roche's chief medical officer and head of global product development. "We are excited to introduce this innovative treatment to individuals in Europe who have PNH. Our goal is to alleviate the challenges associated with managing this condition and improve the quality of life for those affected."
Source: Roche, HemaSphere 2023, 7 (S3) e72750f1, National Library of Medicine